The intricate process of X chromosome inactivation plays a pivotal role in regulating gene expression in females, particularly in the context of genetic diseases such as Fragile X Syndrome and Rett Syndrome. Each female cell carries two X chromosomes, but to avoid an overdose of gene dosage, one chromosome must be inactivated, leading to significant implications for understanding developmental biology and therapeutics. This phenomenon hinges on the action of Xist RNA, a critical molecule that orchestrates the silencing of genes by modifying the chromatin structure surrounding the X chromosome. Advances in this research open doors to potential gene therapy methods that could target these genetic disorders at their root, ultimately offering hope for patients affected by these conditions. As scientists continue to unravel the complexities of X chromosome inactivation, the promise of developing innovative solutions for genetic diseases becomes increasingly tangible.
X chromosome silencing, also known as X inactivation, is a fundamental biological mechanism crucial for balancing gene expression between genders. In females, this process ensures that only one of the two X chromosomes remains active, which is particularly important when considering conditions like Fragile X Syndrome and Rett Syndrome caused by mutations on the X chromosome. The involvement of Xist RNA in this silencing process reveals fascinating insights into how cells can regulate the expression of genes, offering new possibilities for interventions. This research could lead to groundbreaking therapies aimed at alleviating symptoms of genetic disorders through sophisticated gene therapy techniques. By exploring alternative terms and concepts linked to X chromosome regulation and its implications for health, we can deepen our understanding of managing genetic diseases.
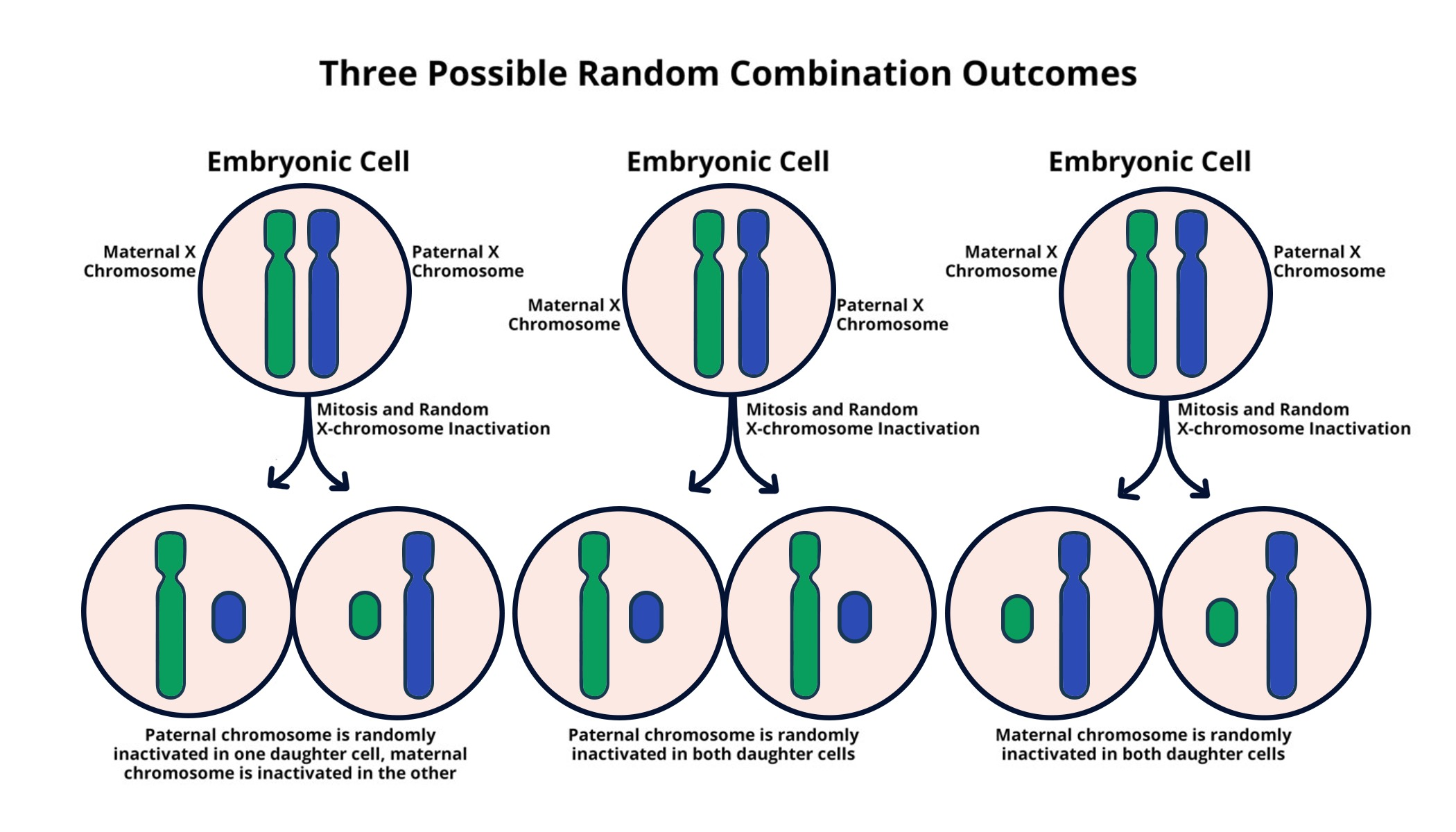
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a critical biological process that occurs in female mammals, where one of the two X chromosomes is randomly silenced to ensure dosage compensation between the sexes. This phenomenon is essential for normal development, as it prevents an overexpression of X-linked genes that could disrupt cellular function. The intricate mechanics of XCI involve a complex interplay of genetic and epigenetic factors, particularly the Xist RNA, which plays a pivotal role in initiating the silencing process. Understanding XCI not only sheds light on fundamental genetic principles but also opens avenues for therapeutic interventions in genetic diseases linked to the X chromosome.
Research on XCI has evolved significantly, particularly with regards to how it contributes to genetic diseases such as Fragile X Syndrome and Rett Syndrome. In the case of these disorders, mutations are often present on the active X chromosome in females, which leads to a lack of functional gene expression. Jeannie Lee’s lab has made substantial strides in decoding the mechanisms behind XCI, demonstrating that the gelatinous substance surrounding chromosomes, metaphorically described as ‘Jell-O’, has profound implications in the accessibility of genes. By manipulating XCI, scientists hope to reactivate silenced genes, potentially providing targeted relief for individuals suffering from these genetic conditions.
The Role of Xist RNA in Gene Therapy
Xist RNA is crucial for the process of X chromosome inactivation, acting as a guide to recruit various molecular components that collectively facilitate the silencing of one X chromosome in females. The significance of Xist RNA extends beyond mere inactivation; it also presents a unique opportunity for gene therapy interventions. By understanding how Xist RNA engages with chromosomal structures, researchers like Jeannie Lee aim to develop strategies that can alter the inactivation process, thereby allowing silenced X-linked genes to be expressed. Such interventions could pave the way for significant advancements in treating genetic disorders linked to the X chromosome.
In conditions like Fragile X Syndrome and Rett Syndrome, the reactivation of silenced genes on the X chromosome is paramount. Gene therapy approaches that leverage the properties of Xist RNA hold promise for restoring healthy gene function by ‘unsilencing’ the inactivated X chromosome. Lee’s research team is investigating methods to optimize Xist-based therapies, focusing on ensuring safety and efficacy before moving into clinical trials. By harnessing the power of Xist RNA, there is potential not only for reversing genetic mutations but also for alleviating symptoms associated with these debilitating diseases.
Therapeutic Promises for Fragile X and Rett Syndromes
Fragile X Syndrome and Rett Syndrome are two prominent genetic disorders linked to mutations on the X chromosome that have devastating effects on cognitive and physical development. Promising therapeutic strategies emerging from research into X chromosome inactivation, particularly those that target silent genes, suggest a potential breakthrough in treating these conditions. By exploring solutions that can reactivate the silenced genes on the inactive X chromosome, scientists are optimistic about fundamentally changing the landscape of treatment for these disorders. Jeannie Lee’s pioneering research into the molecular dynamics of XCI and its influence on gene expression is at the forefront of these developments.
The development of gene therapies for Fragile X and Rett syndromes is critical, as current treatment options focus mainly on symptom management rather than addressing the root genetic causes. The breakthrough discovery that XCI can be manipulated to allow for the expression of previously silent genes might pave the way for a new era of treatment focused on the underlying biology of these disorders. Continued research and clinical trials will be vital for translating these scientific advances into effective therapies that can improve the lives of those affected by these challenging genetic diseases.
Investigating the Gelatinous Substance Around Chromosomes
The metaphor of ‘chromosomal Jell-O’ not only highlights the complexity of X chromosome inactivation but also the unique role that this gelatinous substance plays in maintaining cellular architecture. This viscous environment serves as more than a mere structural component; it acts as a mediator in chromosome behavior and gene expression dynamics. The discovery of how this substance interacts with Xist RNA and facilitates gene silencing has profound implications in understanding genetic diseases, particularly those tied to mutations affecting the X chromosome. Such insights could unlock new approaches in gene therapy.
Researchers are now delving deeper into the properties of this chromosomal ‘Jell-O’. By characterizing its physical state and how it modulates gene accessibility during XCI, scientists aim to craft targeted interventions that could fine-tune gene expression in therapeutic contexts. This innovative approach might enable them to tackle a variety of genetic conditions, transforming our strategic responses to diseases traditionally thought of as having limited treatment options.
Future Directions of Gene Therapy in X-Linked Disorders
The landscape of genetic disease therapy is evolving, particularly with the advent of innovative gene therapies targeting X-linked disorders like Fragile X and Rett Syndromes. The focus on X chromosome inactivation and the roles played by Xist RNA in modulating gene expression provides a promising foundation for novel therapeutic strategies. Soon, clinical trials could emerge that will test the efficacy of various compounds designed to unsilence inactivated genes, offering hope to patients and families affected by these disorders. Within a few years, the marriage of basic scientific exploration and clinical application may lead to tangible advancements in therapy.
Furthermore, researchers are also integrating multidisciplinary approaches, combining molecular genetics with bioengineering to enhance the delivery and efficacy of gene therapies. The goal is to develop treatments that can reach targeted cells within the body with minimal side effects and maximum benefits. This strategic focus on achieving precision in gene therapy for X-linked diseases could ultimately reshape patient experiences and outcomes for many individuals grappling with the challenges of genetic disorders.
Navigating the Mysteries of X-Linked Gene Function
Despite the significant progress made in understanding X chromosome inactivation, many mysteries remain regarding the selective activation and inactivation of genes within this complex genetic landscape. A key observation from Jeannie Lee’s research is that freeing inactivated X chromosomes tends to restore function to mutated genes while leaving healthy counterparts relatively untouched. This fascinating behavior raises questions about the underlying mechanisms that govern gene usage and expression in cells. Delving into this enigma could unveil new insights pertinent to our overall understanding of gene regulation and the complexities of genetic diseases.
Additionally, the distinction in gene expression dynamics poses critical implications for therapeutic applications. If researchers can uncover why certain genes remain unaffected while others are restored, they could develop precise interventions tailored to maximize therapeutic efficacy with minimal risks. This exploration of genetic nuances not only enriches our understanding of X-linked disorders but also exemplifies the intersection of basic research and potential clinical applications, emphasizing a hopeful future for genetic disease management.
The Implications of X Chromosome Research
The advances in understanding X chromosome inactivation have far-reaching implications not only for the treatment of Fragile X Syndrome and Rett Syndrome but for many other genetic disorders linked to this chromosome as well. The insights into how Xist RNA functions and the role of the surrounding chromosomal architecture represent pivotal moments in genetics, highlighting the intertwined relationship between basic research and therapeutic development. As the scientific community continues to unlock the complexities of X chromosome dynamics, it is clear that both basic biology and advanced genetic engineering will play vital roles in shaping future therapies.
Ultimately, the research spearheaded by Jeannie Lee and others serves as a beacon of hope for millions affected by genetic diseases. By elucidating the mechanisms of X chromosome inactivation and exploring how to reactivate essential genes, scientists are working towards potentially revolutionary treatments that could alleviate the burden of these inherited conditions. As we forge ahead in this realm of genetic discovery, the anticipation builds around the prospect of translating laboratory findings into impactful clinical solutions.
The Future of X Chromosome Therapeutics
The prospect of developing effective therapies targeting X-linked diseases has been significantly enhanced by recent advancements in our understanding of the X chromosome. With promising preclinical data emerging from research focused on X chromosome inactivation, gene therapy approaches are now being proposed to selectively reactivate silenced genes. Given the genetic mechanisms involved, researchers are optimistic about the potential for safe and effective treatments that can greatly improve the quality of life for patients affected by disorders like Fragile X and Rett Syndromes.
Looking ahead, the integration of cutting-edge science and advanced clinical practices is likely to accelerate the development and implementation of therapeutics targeting X-linked genetic disorders. Collaboration across disciplines, including molecular biology, genetics, and clinical research, will be key to ensuring swift progress from the laboratory bench to patient care. The ongoing exploration of X chromosome dynamics holds the promise not just for treating genetic diseases but for establishing a new paradigm in genetic medicine.
Frequently Asked Questions
What is X chromosome inactivation and why is it important for genetic diseases?
X chromosome inactivation is a process by which one of the two copies of the X chromosome in females is silenced to prevent overexpression of genes. This is crucial in managing genetic diseases linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome, as it ensures that cells function normally despite having potentially harmful mutations on one of the X chromosomes.
How does X chromosome inactivation relate to disorders like Fragile X Syndrome and Rett Syndrome?
X chromosome inactivation plays a significant role in disorders like Fragile X Syndrome and Rett Syndrome by silencing one copy of the X chromosome. When a mutation is present on the active X chromosome, it can lead to these genetic disorders. Research into unsilencing the inactive X chromosome holds promise for gene therapies aimed at treating these conditions.
What role does Xist RNA play in X chromosome inactivation?
Xist RNA is essential for X chromosome inactivation as it coats the X chromosome and alters the ‘Jell-O’ like substance around it, facilitating the silencing process. This RNA molecule helps create an environment conducive to inactivating the X chromosome, thus preventing the expression of potentially harmful genes linked to genetic diseases.
Can gene therapy be used to treat X-linked genetic diseases stemming from X chromosome inactivation?
Yes, gene therapy has the potential to treat X-linked genetic diseases related to X chromosome inactivation by ‘unsilencing’ the inactive X chromosome. By restoring the function of mutated genes that are otherwise inaccessible due to inactivation, such therapies could provide effective treatments for conditions like Fragile X Syndrome and Rett Syndrome.
What are the potential therapeutic implications of understanding X chromosome inactivation?
Understanding X chromosome inactivation opens up significant therapeutic avenues for genetic diseases caused by mutations on the X chromosome. Insights into this process, particularly how to manipulate it using compounds that interact with Xist RNA, could lead to innovative treatments for conditions like Fragile X Syndrome and Rett Syndrome, improving outcomes for affected individuals.
| Key Points |
|---|
| The X chromosome presents a unique challenge for human cells due to its presence in duplicate in females and only one copy in males. Both sexes do not require twice the X-linked genes. |
| Females inactivate one of their two X chromosomes, a process that has been studied extensively, particularly by Jeannie T. Lee’s lab. |
| The inactivation is facilitated by a gelatinous substance that coats all chromosomes, likened to ‘Jell-O’. |
| Xist, an RNA molecule produced by a gene on the X chromosome, plays a crucial role by altering the properties of this surrounding ‘Jell-O’, leading to gene silencing. |
| The insights gained from this research hold potential for treating X-linked genetic diseases like Fragile X and Rett syndromes by ‘unsilencing’ inactivated genes. |
| Lee’s lab is working on optimizing therapies that could enable access to healthy genes bound within inactivated chromosomes, with plans for clinical trials ahead. |
| While the approach aims to restore gene function with minimal effects on healthy genes, further understanding of the mechanisms is still needed. |
Summary
X chromosome inactivation is a pivotal biological process that allows female mammals to equalize gene dosage between sexes by silencing one of their two X chromosomes. This fascinating mechanism is not only a critical biological concept but also a potential target for innovative therapies aimed at treating genetic disorders linked to the X chromosome. Research spearheaded by Jeannie T. Lee has delved into the intricacies of how this inactivation occurs, revealing the importance of molecules like Xist in modifying chromosomal properties. The implications of this work could lead to groundbreaking treatments for conditions such as Fragile X Syndrome and Rett Syndrome by providing access to normally silenced genes. This ongoing research not only enhances our understanding of chromosome behavior but also opens up new avenues for therapeutic interventions.